How to Determine Which Polymer Is Used for a Molecule
In polyvinyl chloride the repeating unit comes directly from the end-to-end linking of many vinyl chloride molecules. For example if a polymer contains 13.
Of monomer acrylamide 71 Physical Types of HMW Polymers Dry Polymer Cationic anionic non-ionic Molecular weight.

. The composition of the whole sample free of water salts and additives is defined by Acetate butyrate AGU 100. A polymer comprised of three monomer units. Polymer Analysis by NMR.
Polymers often indicate the starting monomer material. Contour length is defined as the length of the polymer if you were to stretch the polymer out in a line. A Depiction of a Floc Formed by a Cationic Polymer Chain Bridging the.
One may describe chain length in terms of polymer average molecular weight which can be defined in several ways. Each vinyl chloride monomer molecule contributes a CH 2 group joined to a CHCl unit by a single bond. A molecule that can be bonded to other identical molecules to form a polymer Dimer.
Therefore an average value must be taken when determining the DP. If Y and Z represent moles of monomer and polymer respectively Z is approximately 10 -5 Y. N sum of CH2 proton integrals of CH2 protons integral per proton value 2079 151874497 869 repeating units n.
1 A number-average molecular weight M n. So you have a new polymer unknown protein or material combination but dont know its molecular weight. The weight-average degree of polymerization is the weighted mean of degrees of polymerization weighted by the weight fractions or the overall weight of.
A polymer ˈ p ɒ l ɪ m ər. The degree of polymerization can be calculated by using the following relationship if a molecular weight of a polymer molecule is known. Natural rubber for example is a polymer that contains large numbers of -CH 2 CCH 3CHCH 2 - units.
If not it has to be dissolved using a. Polymers can have different charges charge densities and molecular weights. It is experimentally determined by measurements of the osmotic pressure of the polymer.
While many people use the term polymer and plastic interchangeably polymers are a much larger class of molecules which includes plastics plus many other materials such as cellulose amber and natural rubber. Due to their broad spectrum of properties both synthetic and natural polymers play essential and ubiquitous roles in everyday life. It can also be used to confirm the target A molecule or molecular complex consisting compound has been made if the reaction pathway is well of two identical molecules linked together Trimer.
Resources you will need. If a polymer is made from more than one type of monomer or has more than a. The number of monomers in a polymer can differ from one chain to the next.
Up to 10 M cationic up to 20M anionic non-ionic. The molecular weight of a polymer is determined by analyzing osmotic pressure data. GPC is an analytical technique that separates molecules in polymers by size and provides the molecular weight distribution of a material.
This exercise should be carried out within a software environment that can generate a best-fit line for an x-y data set. An introductory knowledge of ideal versus non-ideal solution behavior and colligative properties. This polymer is called polyethylene rather than polymethylene -CH 2 - n because ethylene is a stable compound methylene is not and it also serves as the synthetic precursor of the polymer.
Polymers range from familiar synthetic plastics such as polystyrene to. Ns µ s F µs-1 F 4 In defining µs F sums may be truncated at zero since F. Cramer 1946 Zelen and Severs 1964 a few of which find frequent application in their use.
Disregard bond angles just pull it as straight as possible with the constraint being the bond distances. Greek poly- many -mer part is a substance or material consisting of very large molecules or macromolecules composed of many repeating subunits. An easy way to measure molecular weight of your sample is gel permeation chromatography GPC.
One of the challenges polymer scientists face is molecular weight avg. A molecule from which a polymer is made is called a monomer. Thus polytetra uoroetheylene is a polymer made by polymerizing tetra uoroethylene monomers.
Contour length is one of the simplest but also one of the least accurate descriptors of polymer size. If molecular weight of polymer is 10 million the number of monomers in one polymer molecule degree of polymerization n 10000000 71 140850 mol. For example the sth moment µs F of a normalized distribution F n eg either N n or W n defined by µs F Σn s F n 3 is used to compute the averages of the distribution.
Divide chains into series of size ranges and then determine the number fraction x i of each size range where M i represents the mean molecular weight of the size range i and x i. In this method the polymer should be in liquid form. Mn FW end groups FW repeating unit n 5506 7160 4405 869 509.
Polymers that have a positive charge otherwise known as cationic and a high molecular weight are typically used for thickening and dewatering solids separation processes. This single bond is a remnant of the double bond which joined those groups. Chain length determination of their materials.
M is the molecular weight of the polymer DP is the degree of polymerization and the M 0 is the formula weight of the repeating unit. The most accurate method for determine absolute molecular mass of a polymer is vapor pressure osmometry VPO. A polymer may be a natural or synthetic macromolecule comprised of repeating units of a smaller molecule monomers.
While membrane osmometry gel permeation chromatography viscosity analysis and mass spectrometry are typically used for molecular weight determination the techniques can be time consuming inaccurate for the molecular. If a polymer is made from only one type of monomer or if it has a single repeat unit it is called a homopolymer. The most common technique that is used to determine the average molecular weight of a polymer is the viscometry where Ubbelohde viscometer is employed.
Rubber for example is a mixture of polymer chains whose mass differs by a factor of 10 or more but whose average molecular weight is 100000 grams per mole.
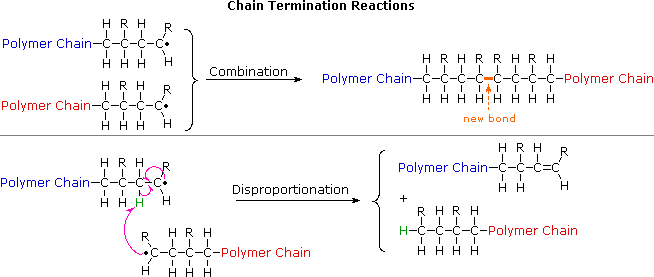
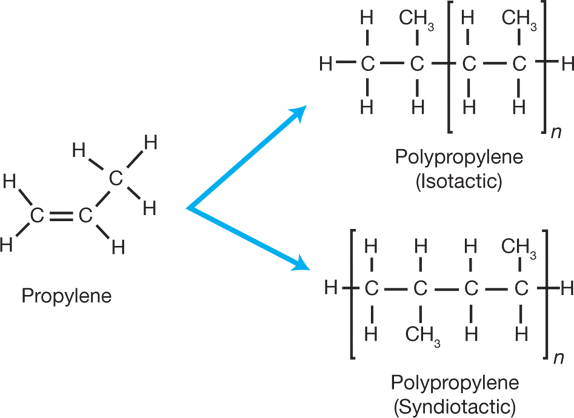
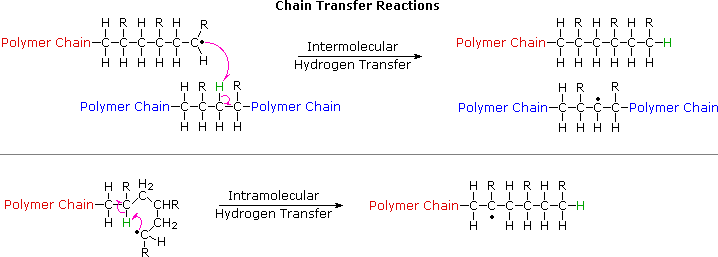
No comments for "How to Determine Which Polymer Is Used for a Molecule"
Post a Comment